Mendeleev and his predecessors based their chart organization according to atomic weight and chemical properties, which were proven to have a strong correlation. However, as more elements were discovered, these two properties did not always accurately predict where the next element should fall; thus that strong relationship between atomic weight and chemical properties didn't always match.
Antonius van den Broeck was the first to hypothesize that there must be another factor that was more influential on the element’s identity other than atomic weight. He had no data however, to support his hypothesis.
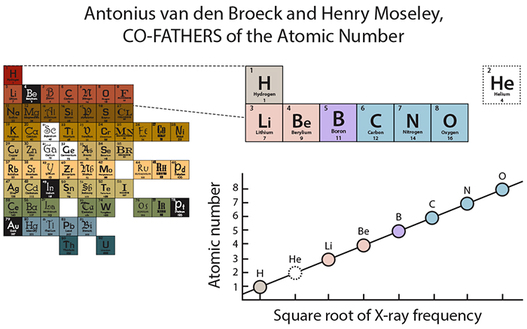
So Henry Moseley devised an experiment based on the result of high-energy, negatively-charged electrons that are shot at elemental substances with each successive chemical element on the table. The X-ray emitted incrementally increased at a consistent rate. So if you look at the graph on the right side, this means that when he shot these electrons at Hydrogen, a certain X-ray emission occurred. Then when he shot it at the next known element, Helium, an equally unique X-ray emission resulted that was at a greater but consistent increase from Hydrogen. When he went on to Lithium, that X-ray emission increased again. When the square root was taken of these individual x-ray emissions, there was a beautiful linear correlation.
This graph is a simplified version explanation, as the emission quantity was NOT one-to-one as I’ve explained it, but the general concept is the same. What he did deduce from this is that another factor within the atom must be giving definition to the element, and as the quantity of that factor changed, so too did the identity of the element. This factor also correlated much more strongly to the organization of chemical property than atomic mass.
What was this factor? Atomic number, or, the number of protons, the positively-charged particle within the atom!
Antonius van den Broeck was the first to hypothesize that there must be another factor that was more influential on the element’s identity other than atomic weight. He had no data however, to support his hypothesis.
So Henry Moseley devised an experiment based on the result of high-energy, negatively-charged electrons that are shot at elemental substances with each successive chemical element on the table. The X-ray emitted incrementally increased at a consistent rate. So if you look at the graph on the right side, this means that when he shot these electrons at Hydrogen, a certain X-ray emission occurred. Then when he shot it at the next known element, Helium, an equally unique X-ray emission resulted that was at a greater but consistent increase from Hydrogen. When he went on to Lithium, that X-ray emission increased again. When the square root was taken of these individual x-ray emissions, there was a beautiful linear correlation.
This graph is a simplified version explanation, as the emission quantity was NOT one-to-one as I’ve explained it, but the general concept is the same. What he did deduce from this is that another factor within the atom must be giving definition to the element, and as the quantity of that factor changed, so too did the identity of the element. This factor also correlated much more strongly to the organization of chemical property than atomic mass.
What was this factor? Atomic number, or, the number of protons, the positively-charged particle within the atom!

 RSS Feed
RSS Feed
