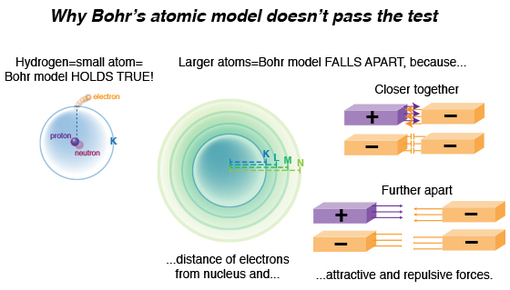
In a simple atom such as hydrogen, where there’s one proton and one electron, quantum mechanics (the physical science used to calculate and analyze the energies and spatial distributions of small particles confined to very small regions of space) calculated hydrogen’s line emission spectrum data (the electron’s absorption or emission of energy whenever it changed energy levels) elegantly. In this simple system, that single electron’s energy emission or absorption was easily quantified, with only a single attractive force acting upon that electron: its one proton.
But let’s take a closer look at the definition of quantum mechanics: small particles, confined to very small regions of space.
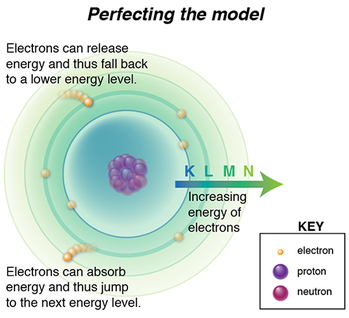
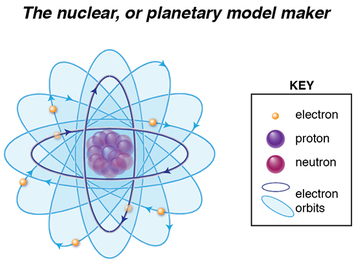
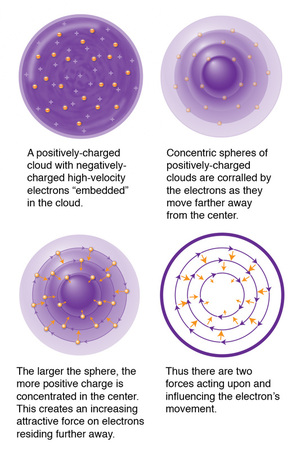
We know atoms are minuscule, but to a proton looking out or an electron looking in, the space between them is MASSIVE. Larger atoms have more electrons, and if, according to Bohr’s model, only a defined number of electrons can occupy certain energy level shells (K,L,M,N, etc), electrons will be further and further away from the protons as they also try to put distance between themselves. Thus, larger atoms do not fit the “confined to small spaces” criterion.
Now, imagine the protons and electrons as magnets of opposing charge. When we bring them close together we feel each pulling toward the other. But when we separate them, that pulling force diminishes! Likewise within an atom, we’ve got two basic factors influencing where the electron is in relation to the PROTON: proximity and charge (not taking into account when an electron is ionized, but we’ll get to that later).
If we bring two negative magnets together, we feel one push away from the other, and as we separate them, that repulsive force diminishes. Once again, two factors influencing where an electron is to another ELECTRON: charge and proximity.
An electron’s energy emission or absorption is affected by all these additional forces, for it has to overcome repellent forces from other electrons nearby, as well as attractive forces of an increasing number of protons. It might be easier to see now why Bohr’s model starts to fall apart, right?



 RSS Feed
RSS Feed
