1) Why are viruses so destructive and even deadly?
2) What then, does get rid of a virus?
3) Viral infections, once begun, often progress to bacterial infections, but why?
I’m NOT going to get to those questions just yet, because a suggestion was made by a loyal fan (can I call you that, Don?) to cover what an antiviral is, as opposed to an antibiotic. This makes sense given that antiviral drugs for the flu are available, and you may have been prescribed that in lieu of an antibiotic. So, let’s see what the difference is between an antibiotic and an antiviral, before we get onto our other questions.
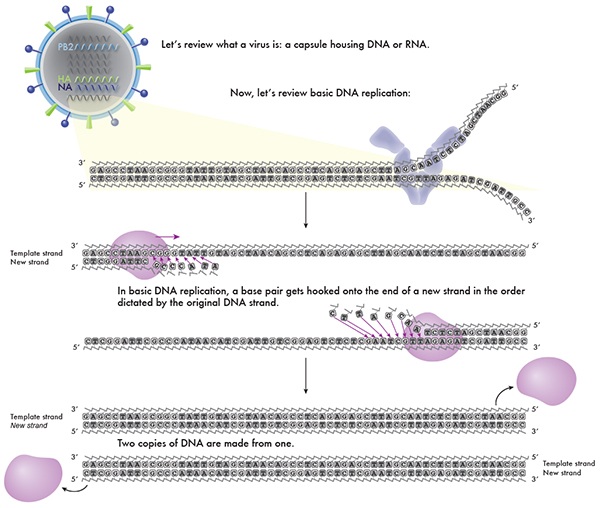
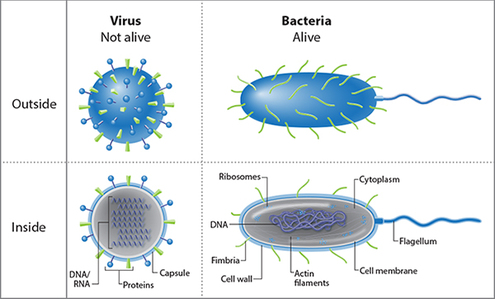
In this graphic, we go back to the basics to find a strategy to undermine a virus. A virus is nothing more than a capsule or vehicle for its DNA/RNA. And we learned from our antibiotic post that antibiotics target specific molecules within a cell that disrupt its normal cellular functioning. Well, with a virus, there’s no molecule to disrupt, so antibiotics are actually useless against it.
So what do scientists target against a virus? 1) the capsule, and 2) the DNA/RNA.
A virus’ one and only mission is to make more copies of itself. But since it doesn’t have the infrastructure to do so, it takes over a cell in order to use all the special enzymes and building materials to for its own goal: to copy its DNA/RNA and make new virus capsules to house those new copies. If this is the case, can you guess what the antiviral might target in order to stop viruses from making copies of itself?

 RSS Feed
RSS Feed
