We’re now going to cover the elemental families that comprise the table. You can easily find the family descriptions online, so I’ll try to look at this from a different angle.
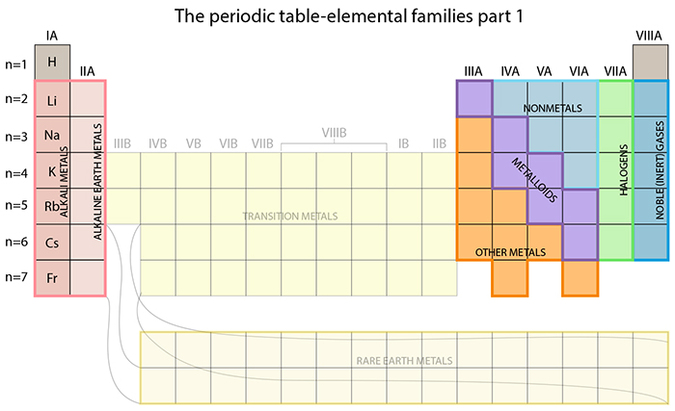
The chemical properties of an element are determined mainly by how many valence electrons it has. This factor also greatly dictates the organization within the table of the elements because it dictates how that element will react in the presence of other elements. So let’s look at the elements of the left-most column, IA: these elements have one electron in their outermost shell. This lone electron occupies the outermost orbital by itself, and is therefore highly susceptible to being taken away by elements that are one electron away from having a completely full outermost shell of 8 electrons (Ionic bonding; octet rule). What about the fact that more and more protons are present as we go down the group? We’ve learned that that collective proton force prevents electrons from being snatched. But remember, these electrons get farther away from the proton forces as more shells are added, so physically, they’re not influenced so much by the protons.
The fact that these elements very easily lose their lone valence electron means that they REACT similarly to each other when interacting with other elements. That’s what gives them SIMILAR chemical properties and hence what makes them logically part of the same group.
Why would elements in group IA allow this, for as a result it now has an unbalanced charge of +1? Here’s the beautiful part: once that lonely electron is gone, that original, outermost shell is now empty. For all elements but Hydrogen, “underneath” that now-empty shell is a full, and therefore stable, shell! It’s happy to lose that one electron in order to reveal the full shell underneath! Now it effectively has a full valence shell.
SO, did anyone catch the “A” designation after the group “I” part? Next post!
The chemical properties of an element are determined mainly by how many valence electrons it has. This factor also greatly dictates the organization within the table of the elements because it dictates how that element will react in the presence of other elements. So let’s look at the elements of the left-most column, IA: these elements have one electron in their outermost shell. This lone electron occupies the outermost orbital by itself, and is therefore highly susceptible to being taken away by elements that are one electron away from having a completely full outermost shell of 8 electrons (Ionic bonding; octet rule). What about the fact that more and more protons are present as we go down the group? We’ve learned that that collective proton force prevents electrons from being snatched. But remember, these electrons get farther away from the proton forces as more shells are added, so physically, they’re not influenced so much by the protons.
The fact that these elements very easily lose their lone valence electron means that they REACT similarly to each other when interacting with other elements. That’s what gives them SIMILAR chemical properties and hence what makes them logically part of the same group.
Why would elements in group IA allow this, for as a result it now has an unbalanced charge of +1? Here’s the beautiful part: once that lonely electron is gone, that original, outermost shell is now empty. For all elements but Hydrogen, “underneath” that now-empty shell is a full, and therefore stable, shell! It’s happy to lose that one electron in order to reveal the full shell underneath! Now it effectively has a full valence shell.
SO, did anyone catch the “A” designation after the group “I” part? Next post!

 RSS Feed
RSS Feed
