|
I know I’ve belabored this point about electron orbitals, but it’s really an important point in chemistry.
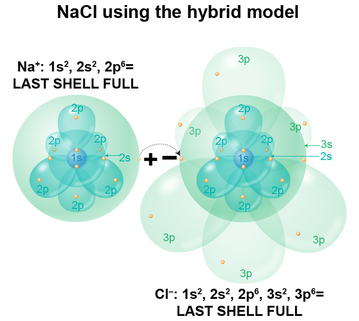
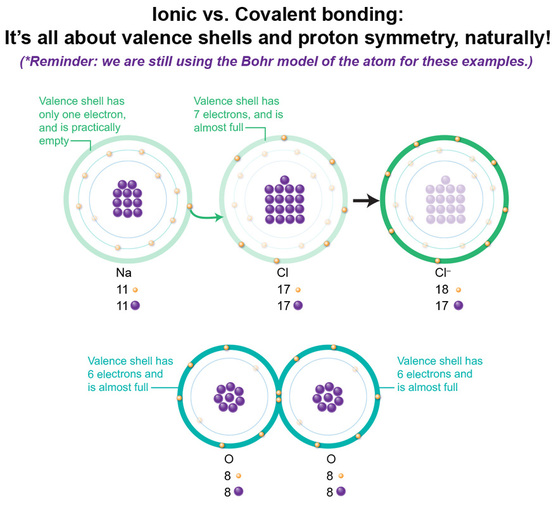
In the salt compound, an Na–PLUS ion has 10 electrons, one fewer than the Na atom, so if we fill the clouds one by one following Aufbau's principle, we get 1s2 and 2s2 (spherical clouds), 2p6 (dumbell cloud). The last, outermost shell is now a full one. In the Cl–MINUS ion we have 18 electrons, one more than the Cl atom, so if we fill the clouds one by one, we get 1s2 and 2s2 (spherical clouds), 2p6 (dumbell cloud), 3s2 (spherical cloud) and 3p6 (dumbell cloud). The last, outermost shell is now a full one. See the pattern? The transfer of Na’s 3s1 electron into the Cl’s 3p5 shell gives both atoms a completely-filled outermost shell. This is why this configuration occurs because suddenly, each atom is entirely stable in compound form, with the positive ion of Na now bonded to the negative ion of Cl. Thus we get the compound salt. Next, we’ll do this all over again for O2, the oxygen molecule! We’ve now "completed" the history of the development of the atomic model with Heisenberg. With his clarification that electron orbitals aren’t so much a trajectory or travel path, but rather a cloud of probability in which a single electron can be found, let’s apply THIS model to NaCl, instead of using the incorrect-but-easier-to-understand Bohr model.
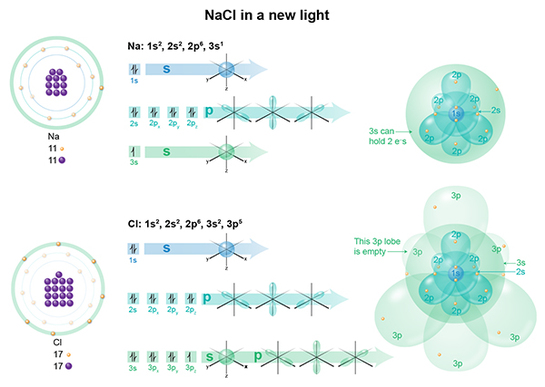
This image shows the Bohr model of each atom on the left, its electron configuration in the middle, and then its application of the electron cloud model on right. I think you can immediately see why Bohr’s model, for certain atoms, is much easier to teach with, than the cloud model on right. But let’s walk through this. In a Na atom, we have 11 electrons, so if we fill the clouds one by one following Aufbau's principle, we get 1s2 and 2s2 (spherical clouds), 2p6 (dumb bell cloud), and 3s1 (spherical cloud). This last cloud is only half-filled, as 3s2 can hold 2 electrons. In a Cl atom, we have 17 electrons, so if we fill the clouds one by one, we get 1s2 and 2s2 (spherical clouds), 2p6 (dumbell cloud), 3s2 (spherical cloud) and 3p5 (dumbell cloud). This last cloud is almost filled, as 3p6 can hold 6 electrons. There's one lone lobe BEGGING to be filled, while in Na, there's one lonely electron. What would happen if that electron were to pick up and move to the Cl suburb? Before we get to the Periodic Table discussions, let's make a few more points about electrons.
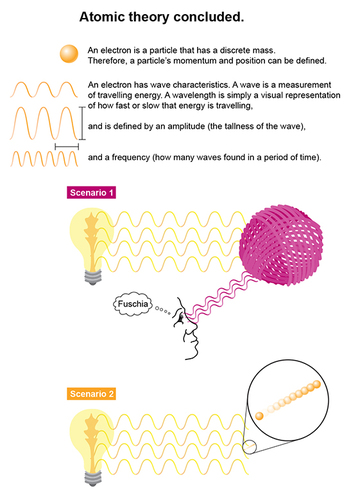
Louis de Broglie was the first scientist to deduce that an electron has both particle and wave properties. The electron's minute size IS its defining characteristic, and why we cannot define both its momentum and exact position in space simultaneously. If we humans try to SEE where a ball of yarn (see scenario 1), for instance, is, we can’t see it unless we turn on a light. When the light waves reach the ball of yarn, they bounce off that ball and reflect back to our eye, which then perceives the color and shape of the ball. The yarn ball is unaffected. With a teeny subatomic particle like the electron, turning on that light sends this wave of energy that affects that electron’s trajectory (scenario 2), and hence its momentum and position. Though this “observer effect” theory is separate from the Heisenberg Uncertainty Principle–which states that both position and momentum potential cannot be calculated simultaneously–it helps to explain why this is so. The electron can receive an energy transfer from the light wave of the light we use to see it, which then affects its momentum potential. Likewise, the light wave's force can change the electron’s position. What does this ultimately mean? It means that any discrete “orbit” that previous atomic models have used to define location cannot be true! And thus, the idea of electron probability clouds replaced the electron orbit theory. Final words about those clouds next post! I’m interrupting myself–again–to talk about an infographic from a company blog that a friend originally shared on facebook a while ago. I thought I’d use my own thought processes as an example as to how I, versus a trained scientist (e.g., my sister), digested this graphic.
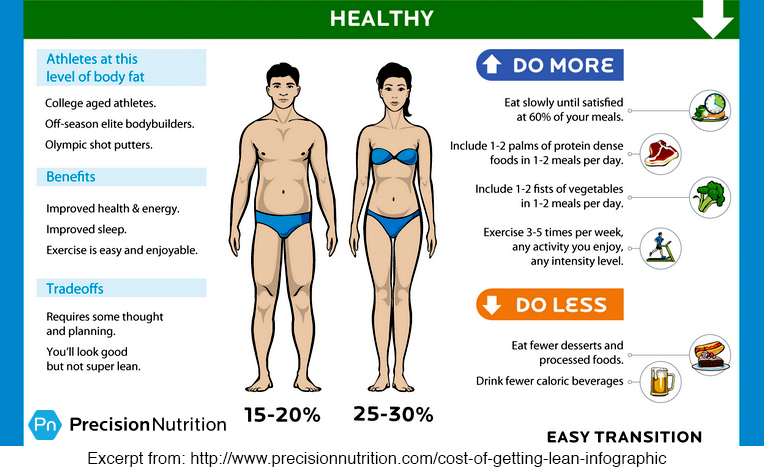
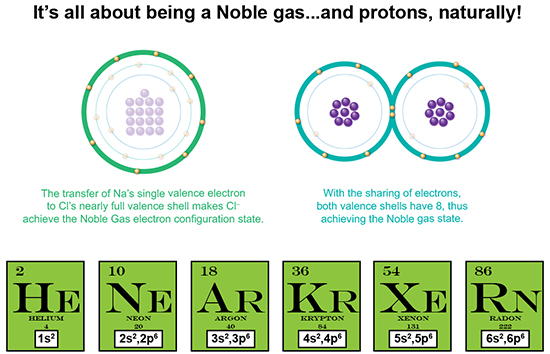
This infographic image is one panel from a series that depicts the subsequent trade-offs that an individual must adhere to in order to gain the body image to which s/he strives. Before even looking at the company that produced the graphic, I read through the first couple of paragraphs (noting its many grammatical errors), and then skimmed through the next few paragraphs to try to get a sense of what the blog was advocating. I ended up believing the blog because at first glance, it seemed to merely imply that with extreme goals (of any kind), there can be adverse consequences, a concept I rabidly support. My initial reactions: 1) What a great graphic! Layout and information structure stay the same to emphasize the difference from one screen to the next. Graphics are devoid of superfluous imagery. 2) Graphic visually correlates the changes in physique with increasing mental, social and physiological demands necessary to achieve that physique. 3) THIS GRAPHIC IS WELL THOUGHT OUT. I APPROVE AND ACCEPT IT AS AUTHORITY. Then I showed it to my sister, who has a Ph.D. in Nutritional Biochemistry, and here are her initial impressions: 1) The company behind this is a health coaching company, so, there may be a hidden agenda/advocacy behind publishing this graphic. 2) Overall, this graphic series are interesting in its incorporation of the social, psychological and physiological aspects that could impact upon the returns provided by increasing levels of fitness. Will the individual find the trade-offs worth it? 3) Interesting in the holistic approach they present. A trained scientist is interested in results, but more so, immediately asks more questions about the information presented like: who’s making the claim? What do they gain from publishing these results? Where’s the data? It’s NORMAL to believe what you already believe in, and poo-poo what you don’t. So, this company is a nutrition/fitness coaching company. Their goal is to help you achieve your fitness goals for a fee. That's where I get a tad suspicious. But what I do appreciate in what they've created is the reality of how multi-factorial physical fitness maintenance is, and further, to possibly make you feel better about why you may not be able to achieve that 6-pack. Most of all, whereas fitness should not be an optional goal, we don't all have to be elite athletes to be deemed healthy and fit. A healthy lifestyle is ALWAYS a great goal, but a healthy lifestyle ALSO means one that is full of joy and anticipation of doing things we love to do. And if that includes dining with friends and trying new restaurants like it does for me, that's an important consideration that for me is not worth taking off the table for that 6–pack. The number of Bohr model valence shell electrons in each of these elements is the magic number 8. This number represents the fact that the lowest energy orbital, the __s2__p6, is completely full, while the next higher energy orbital is completely empty. This “Octet Rule” describes the desired closed-shell state which is the most stable state for an atom.
We’ve also now just described what a noble gas is. Being entirely stable, it is non-reactive, which means it doesn’t generally react to form ionic or covalent bonds with other atoms, because it already has the full shell. Therefore, it doesn’t need to snatch, give away, or share electrons to achieve that full or closed-shell state. The “Octet Rule” also demonstrates the distinction made between the valence shell and valence electrons. Back when my niece was confused by my saying the n=3 shell had the capacity for 18 electrons, and she was taught that n=3 holds 8 electrons, it is because she’s learned the Octet Rule. The elements near Neon, the noble gas that naturally has the n=2, closed-shell state of 2s2,2p6, will do what they can to achieve this same electron configuration, and thus achieve ultimate stability. These elements are: N, O, F, Na, Mg and Al. However, n=3 has 3 “l”-shaped orbitals: s, p and d, where s has a capacity to hold 2 electrons, p has the capacity of 6, and d has a capacity of 10. What do you get when you add 2+6+10? 18! Finally, what determines if an electron is transferred or shared? Take a look at the disparity in proton number between the elements forming a compound. For instance, Na has 11 protons whereas Cl has 17. That’s a difference of 6 protons, meaning that there is a greater positive attractive force exerted by Cl than by Na. And therefore, Cl has a more attractive power to “snatch” that single electron away, as well as keep hold of its own electrons. So, how about two oxygen atoms? Each has 8 protons, meaning each exerts equal force on the other atom’s valence electrons. So they come together and share. Isn’t that just so elegant? Next post let’s tackle something even more elegant, the Periodic Table! We have now discerned that while all electrons are created equal, only certain electrons are involved in forming chemical compounds, with their locations within the atom being key to...everything!
So, 2 important factors influencing whether a compound is formed through ionic or covalent bonding are: 1) Number of valence shell electrons. The transfer (ionic bonding) or the sharing (covalent bonding) of electrons that leads to chemical formation depends on how close to a full valence shell each element is. If one element's valence shell is almost full, it'll be more likely to overpower an electron that is all by itself in another element's valence shell. So it happens in Na, where there's only one valence electron, whereas in Cl, there are 7, one away from a full shell. But if both elements have similar numbers of valence electrons as in oxygen, neither has a distinct advantage in being able to snatch the electrons needed to get to 8. But, both may achieve the complete 8 if they share. 2) Total number of protons for each element. There are two factors to re-visit: the distance the electrons are from the protons, and the number of protons. Collectively, the more protons there are, the more attractive force they will exert on the nearest electrons. So in elements with a lot of protons, those nearest are really hard to rip away, as occurs in NaCl where Cl has significantly more protons than Na. Both use the 3rd shell for their valence shell, so each set of valence electrons are about the "same" distance away from their respective protons' force. Yet with Na, there are fewer protons, and thus less attractive force imposed upon that valence electron, whereas in Cl, there are more protons and thus more attractive force on its valence electrons. So Na's valence electron becomes somewhat easy to steal. If both elements have similar attractive force power, the elements share rather than steal, as in our example of oxygen. This snatching and sharing is all to get to 8 valence electron, or complete shell status. But why 8? Didn't we learn that the 3rd orbital holds a maximum of 18 electrons? Think Noble gas. We'll get back to this point. |
The purpose of this blog is to explore more effective and exciting ways to communicate science. Archives
June 2017
|

 RSS Feed
RSS Feed
