I know I’ve belabored this point about electron orbitals, but it’s really an important point in chemistry.
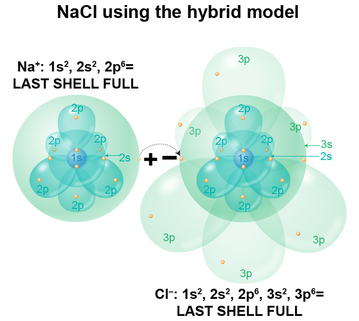
In the salt compound, an Na–PLUS ion has 10 electrons, one fewer than the Na atom, so if we fill the clouds one by one following Aufbau's principle, we get 1s2 and 2s2 (spherical clouds), 2p6 (dumbell cloud). The last, outermost shell is now a full one.
In the Cl–MINUS ion we have 18 electrons, one more than the Cl atom, so if we fill the clouds one by one, we get 1s2 and 2s2 (spherical clouds), 2p6 (dumbell cloud), 3s2 (spherical cloud) and 3p6 (dumbell cloud). The last, outermost shell is now a full one.
See the pattern? The transfer of Na’s 3s1 electron into the Cl’s 3p5 shell gives both atoms a completely-filled outermost shell. This is why this configuration occurs because suddenly, each atom is entirely stable in compound form, with the positive ion of Na now bonded to the negative ion of Cl. Thus we get the compound salt.
Next, we’ll do this all over again for O2, the oxygen molecule!
In the salt compound, an Na–PLUS ion has 10 electrons, one fewer than the Na atom, so if we fill the clouds one by one following Aufbau's principle, we get 1s2 and 2s2 (spherical clouds), 2p6 (dumbell cloud). The last, outermost shell is now a full one.
In the Cl–MINUS ion we have 18 electrons, one more than the Cl atom, so if we fill the clouds one by one, we get 1s2 and 2s2 (spherical clouds), 2p6 (dumbell cloud), 3s2 (spherical cloud) and 3p6 (dumbell cloud). The last, outermost shell is now a full one.
See the pattern? The transfer of Na’s 3s1 electron into the Cl’s 3p5 shell gives both atoms a completely-filled outermost shell. This is why this configuration occurs because suddenly, each atom is entirely stable in compound form, with the positive ion of Na now bonded to the negative ion of Cl. Thus we get the compound salt.
Next, we’ll do this all over again for O2, the oxygen molecule!

 RSS Feed
RSS Feed
