This is from the seminal publication of 1869, in which Dmitri Mendeleev first proposed THE predecessor to our periodic table’s organization.
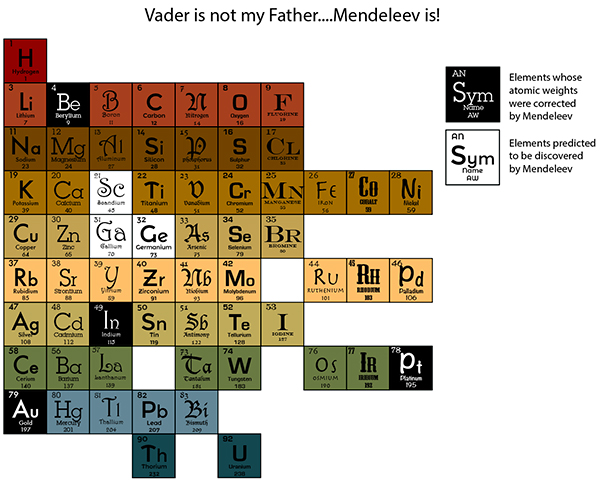
Here is my favorite illustration of this series.
As you see, this chart is beginning to resemble our current periodic chart. The color and font variations are merely MY stylistic changes to remind us of the importance of the row and column groupings. Mendeleev, however, did more than just classify and group elements; his table allowed him to see such strong patterning of physical and chemical properties, that he was bold enough to call into question certain elements’ accepted atomic weights. Their lack of correspondence to the periodicity shown in the table gave the weight to his argument, and, with further investigation, he was proven correct. Not only did his insightful groupings correct atomic weights of Beryllium, Indium, Gold and Platinum, for example, but, it also allowed him to predict the existence of several unknown elements and their behavioral characteristics as governed by his chart.
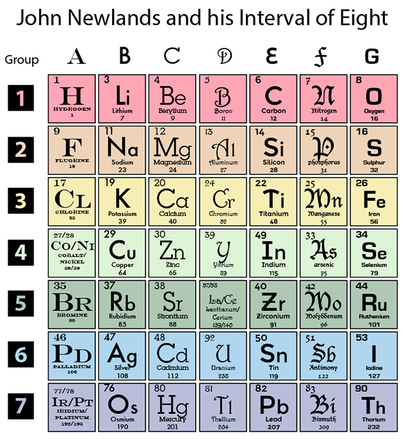
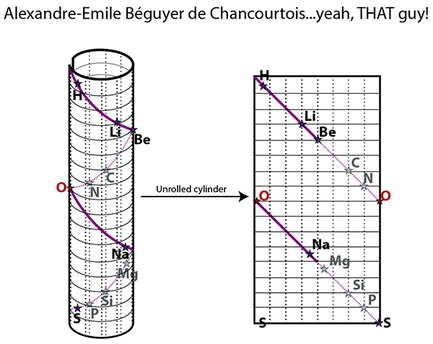
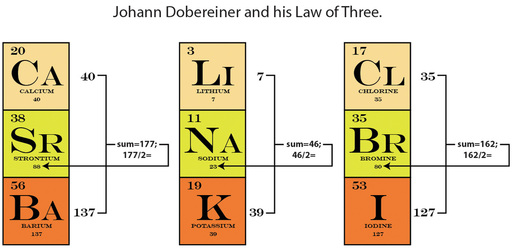
IT SHOULD BE NOTED, that in 1864, 5 years prior to the publication of Mendeleev’s paper, that a Chemist name Julius Lothar Meyer had come to very similar conclusions about the periodic nature of elements. However, it wasn’t until 1870, a year after, that he formally published his version of the periodic chart! Who was truly the first frankly comes down to a matter of the publication race, a reality of the science world, even back then!

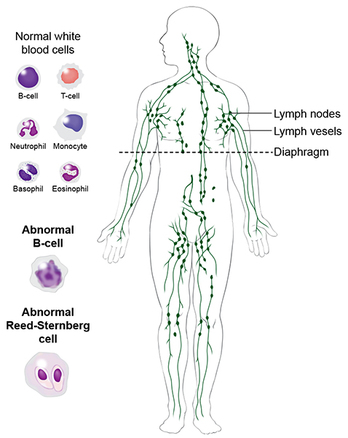
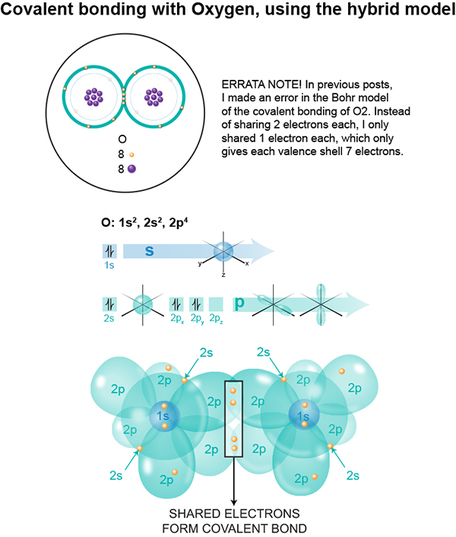
 RSS Feed
RSS Feed
