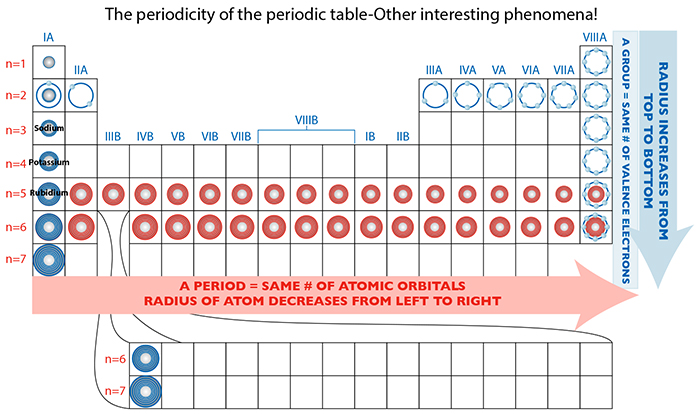
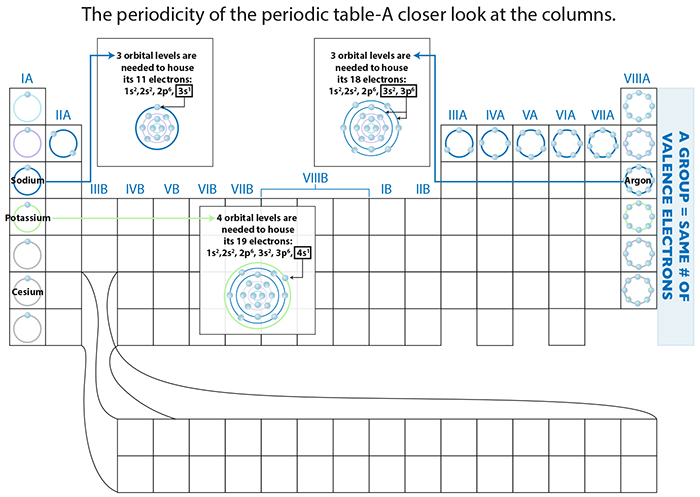
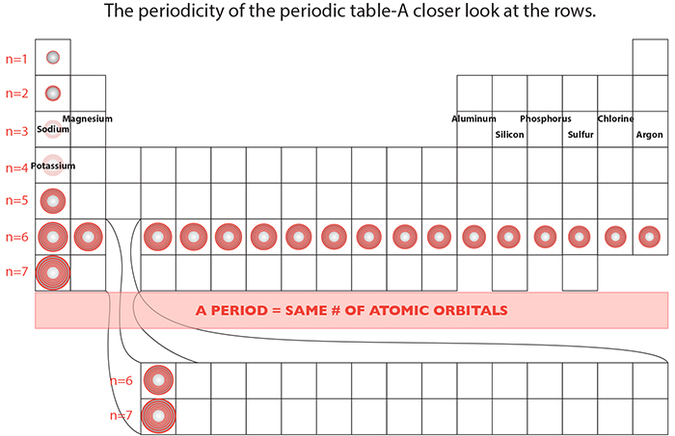
In the case of the decreasing atomic radii for periods, as one more proton is added, a greater positive force per additional proton is exerted on the negligible negative force from the additional electron. This collective attractive force contributed by a proton particle of more substantial mass than its electron counterpart, is capable of pulling the electrons closer to its center, thereby contracting the overall dimension of the atom.
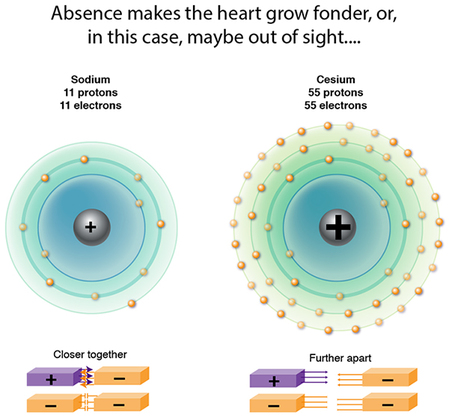
So, why do the atomic dimensions increase in groups? Significant increases in proton attractive force are occurring, so yes, collective proton charge is also increasing...but so too are the repulsive forces among the very crowded electrons. And take a look at the figure: there is an increasing DISTANCE from the nucleus to the farthest flung electrons, effectively diminishing that proton attractive force. The repulsive forces therefore take over, the electrons spread out as much as possible, and to accommodate this the atomic dimension also expands!

 RSS Feed
RSS Feed
