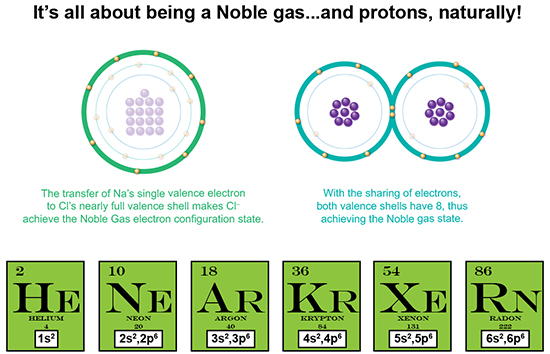
The number of Bohr model valence shell electrons in each of these elements is the magic number 8. This number represents the fact that the lowest energy orbital, the __s2__p6, is completely full, while the next higher energy orbital is completely empty. This “Octet Rule” describes the desired closed-shell state which is the most stable state for an atom.
We’ve also now just described what a noble gas is. Being entirely stable, it is non-reactive, which means it doesn’t generally react to form ionic or covalent bonds with other atoms, because it already has the full shell. Therefore, it doesn’t need to snatch, give away, or share electrons to achieve that full or closed-shell state.
The “Octet Rule” also demonstrates the distinction made between the valence shell and valence electrons. Back when my niece was confused by my saying the n=3 shell had the capacity for 18 electrons, and she was taught that n=3 holds 8 electrons, it is because she’s learned the Octet Rule. The elements near Neon, the noble gas that naturally has the n=2, closed-shell state of 2s2,2p6, will do what they can to achieve this same electron configuration, and thus achieve ultimate stability. These elements are: N, O, F, Na, Mg and Al. However, n=3 has 3 “l”-shaped orbitals: s, p and d, where s has a capacity to hold 2 electrons, p has the capacity of 6, and d has a capacity of 10. What do you get when you add 2+6+10? 18!
Finally, what determines if an electron is transferred or shared? Take a look at the disparity in proton number between the elements forming a compound. For instance, Na has 11 protons whereas Cl has 17. That’s a difference of 6 protons, meaning that there is a greater positive attractive force exerted by Cl than by Na. And therefore, Cl has a more attractive power to “snatch” that single electron away, as well as keep hold of its own electrons. So, how about two oxygen atoms? Each has 8 protons, meaning each exerts equal force on the other atom’s valence electrons. So they come together and share.
Isn’t that just so elegant? Next post let’s tackle something even more elegant, the Periodic Table!
We’ve also now just described what a noble gas is. Being entirely stable, it is non-reactive, which means it doesn’t generally react to form ionic or covalent bonds with other atoms, because it already has the full shell. Therefore, it doesn’t need to snatch, give away, or share electrons to achieve that full or closed-shell state.
The “Octet Rule” also demonstrates the distinction made between the valence shell and valence electrons. Back when my niece was confused by my saying the n=3 shell had the capacity for 18 electrons, and she was taught that n=3 holds 8 electrons, it is because she’s learned the Octet Rule. The elements near Neon, the noble gas that naturally has the n=2, closed-shell state of 2s2,2p6, will do what they can to achieve this same electron configuration, and thus achieve ultimate stability. These elements are: N, O, F, Na, Mg and Al. However, n=3 has 3 “l”-shaped orbitals: s, p and d, where s has a capacity to hold 2 electrons, p has the capacity of 6, and d has a capacity of 10. What do you get when you add 2+6+10? 18!
Finally, what determines if an electron is transferred or shared? Take a look at the disparity in proton number between the elements forming a compound. For instance, Na has 11 protons whereas Cl has 17. That’s a difference of 6 protons, meaning that there is a greater positive attractive force exerted by Cl than by Na. And therefore, Cl has a more attractive power to “snatch” that single electron away, as well as keep hold of its own electrons. So, how about two oxygen atoms? Each has 8 protons, meaning each exerts equal force on the other atom’s valence electrons. So they come together and share.
Isn’t that just so elegant? Next post let’s tackle something even more elegant, the Periodic Table!

 RSS Feed
RSS Feed
