In life, there are always exceptions to a rule. And though we have these rather elegant patterns that dictate the organization of our periodic table, we also have a gigantic asterisk to denote an exception to these patterns. This exception is the transition metals, as their reactive electrons are mercurial!
We‘ve been stressing the importance of valence electrons, those in the outermost shells of the atom, because these are the ones that are most prone to being snatched, to snatching, or, to share, all in the pursuit of obtaining the full valence shell “octet” status.
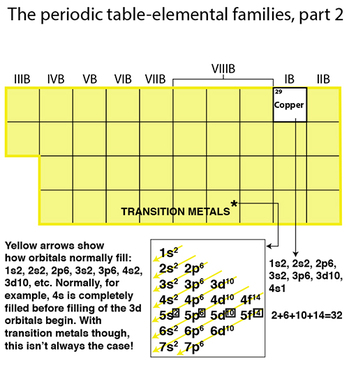
Transition metals, however, are curious in that the general rule for filling up shells one by one doesn’t really apply. In fact, the second to the last shell tends to get filled before the valence shell. Look at copper. One would think the filling order would be: 1s2, 2s2, 2p6, 3s2, 3p6, 4s2, 3d9, according to the rule. Actually the last shells as filled this way: 3d10, 4s1!
Each shell level (n=1, 2, 3, 4 etc) can hold a maximum of 32 electrons, but often due to optimizing spread as a result of electron-electron repulsive forces, proton attraction pulling them closer to the center, and electron spin, the electrons don’t often max out that 32....Unless it’s a transition metal! In this case, often this second to last outer shell gets “over-stuffed,” and sometimes these inner-shell electrons are involved in reactions! This is why these elements end up with a “B” designation in their groups, because frankly, they do NOT fit into the patterns that the “A” groups do.
Thanks for sticking with this journey. I hope it gave you more appreciation for the Periodic Table and the evolution through which it has gone!
We‘ve been stressing the importance of valence electrons, those in the outermost shells of the atom, because these are the ones that are most prone to being snatched, to snatching, or, to share, all in the pursuit of obtaining the full valence shell “octet” status.
Transition metals, however, are curious in that the general rule for filling up shells one by one doesn’t really apply. In fact, the second to the last shell tends to get filled before the valence shell. Look at copper. One would think the filling order would be: 1s2, 2s2, 2p6, 3s2, 3p6, 4s2, 3d9, according to the rule. Actually the last shells as filled this way: 3d10, 4s1!
Each shell level (n=1, 2, 3, 4 etc) can hold a maximum of 32 electrons, but often due to optimizing spread as a result of electron-electron repulsive forces, proton attraction pulling them closer to the center, and electron spin, the electrons don’t often max out that 32....Unless it’s a transition metal! In this case, often this second to last outer shell gets “over-stuffed,” and sometimes these inner-shell electrons are involved in reactions! This is why these elements end up with a “B” designation in their groups, because frankly, they do NOT fit into the patterns that the “A” groups do.
Thanks for sticking with this journey. I hope it gave you more appreciation for the Periodic Table and the evolution through which it has gone!

 RSS Feed
RSS Feed
