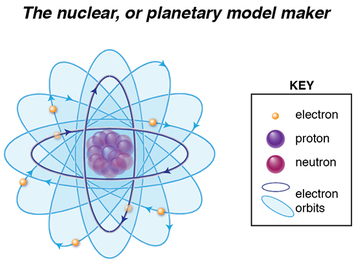
Rutherford was a student of JJ Thomson’s. Known as a creative and collaborative scientist, Rutherford evolved Thomson’s plum pudding model to what is known as the nuclear or planetary model, where most of the atom’s space is actually empty, with some type of positively-charged subatomic particles concentrated in the center. His model differed from Thomson’s model in two key points: 1) there was no positively-charged soup or cloud in which the negatively-charged electrons were embedded, and 2) The positive charge was concentrated centrally, rather than spread uniformly within the atom.
Rutherford had performed experiments leading to his nuclear theory, where he observed that alpha particles that were shot through a gas don’t go straight through. Instead, they got deflected at an angle, therefore there must be something within the atom that repelled them off their linear course.
Rutherford also made important postulations about the nature of elements, which lead to the organization of the periodic chart.
Rutherford had performed experiments leading to his nuclear theory, where he observed that alpha particles that were shot through a gas don’t go straight through. Instead, they got deflected at an angle, therefore there must be something within the atom that repelled them off their linear course.
Rutherford also made important postulations about the nature of elements, which lead to the organization of the periodic chart.

 RSS Feed
RSS Feed
