I know these last few posts have started to go beyond the level of comfort, at least for me, but I invite you to bear with me and continue along this electron adventure. Because it's about to get worse!
I have presented the complex scenario that is the inner life of an atom. Furthermore, I have focused on how complex it is to BE an electron. Now, we finally get to the heart of the matter!
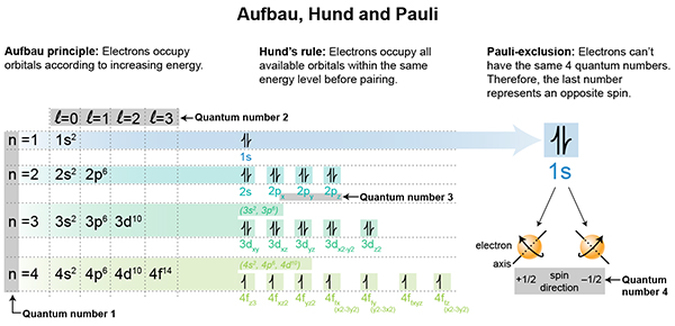
The calculation of an electron’s possible whereabouts boils down to 4 quantum numbers that are assigned to each electron, whereby no electron can have the same four numbers! What exactly are these numbers and what do they mean?
Quantum number 1 is the energy level of the electron and is designated as the “n” number. The higher the number, the higher the energy that electron possesses.
Quantum number 2 is the orbital angular momentum and is designated by the “l” number. In visual terms, it describes the shape of the cloud in which the electron is generally found.
Quantum number 3 is the magnetic quantum number, designated as “ml”. In visual terms it refers to the different sub-shells within an energy shell (hint, has to do with valence electrons. We will get into that in a future post).
Quantum number 4 is the spin magnetic quantum number “ms”. This describes the electron’s spin around its own axis, so as it’s moving about, it’s also spinning, like the Earth does, around its own axis.
We’re going to take this graphic APART and ADD to it. But you may be wondering, WHY are we spending SO much time on electrons? Can anyone answer that? Because it’s frankly the crux of chemistry, and life as we know it!I
I have presented the complex scenario that is the inner life of an atom. Furthermore, I have focused on how complex it is to BE an electron. Now, we finally get to the heart of the matter!
The calculation of an electron’s possible whereabouts boils down to 4 quantum numbers that are assigned to each electron, whereby no electron can have the same four numbers! What exactly are these numbers and what do they mean?
Quantum number 1 is the energy level of the electron and is designated as the “n” number. The higher the number, the higher the energy that electron possesses.
Quantum number 2 is the orbital angular momentum and is designated by the “l” number. In visual terms, it describes the shape of the cloud in which the electron is generally found.
Quantum number 3 is the magnetic quantum number, designated as “ml”. In visual terms it refers to the different sub-shells within an energy shell (hint, has to do with valence electrons. We will get into that in a future post).
Quantum number 4 is the spin magnetic quantum number “ms”. This describes the electron’s spin around its own axis, so as it’s moving about, it’s also spinning, like the Earth does, around its own axis.
We’re going to take this graphic APART and ADD to it. But you may be wondering, WHY are we spending SO much time on electrons? Can anyone answer that? Because it’s frankly the crux of chemistry, and life as we know it!I

 RSS Feed
RSS Feed
