Up to 8 electrons can occupy the next orbital shell. Though one electron wants to remain as far away from the next, there’s another electron in the way that is also dealing with repelling and being repelled. That is, instead of 1 electron exerting on and experiencing force from 1 electron as in the 1st shell, this 2nd shell electron will be exerting a repelling force, as well as having force exerted upon it, by 7 other electrons.
What's more, the shells aren’t isolated from one another! The 1st shell electrons exert force on the 2nd shell electrons etc. AND likewise those 2nd shell electrons are repelling the 1st shell electrons. So at this point, all 10 electrons are exerting and experiencing repelling forces from numerous electrons in their own orbital as well as from other occupied orbitals. In the lower left figure, I imagine visually what this might look like, using dotted lines of equal length to represent the equal distances represented by the equal forces of repulsion that each electron is experiencing and exerting. So in my map, I theorize how all 10 electrons might orient themselves to be most optimal with respect to another.
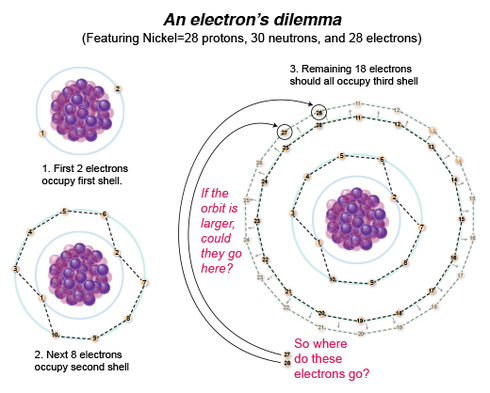
NOW, let’s fill in orbital shell 3, which can hold up to 18 electrons. In the right-hand figure, I try the mapping thing again, spreading these electrons out equally within their orbit, but can only fit 16 of the 18 remaining electrons into the orbital. If I simply increase the radius of the orbit, I can get all 18 electrons in the orbit! Et voila!
But no, this does not happen in reality. Why?
Before I move on, I want to make a clarification on valence shell capacity: 18 is the maximum occupancy of electrons that the 3rd shell can hold, but if that 3rd shell is the valence, or outermost shell, one is taught in school that the max. number it can hold is 8. While orbital "shells" are a concept that is no longer valid, for now, think of these shells as having "sub-shells," with the outermost sub-shell being the valence shell. Thus they determine that atom's chemical properties since they are the ones that interact with the valence electrons of another atom/s. Basically, it gets so much more complicated from here, and what I have been trying to do the last few posts is explain some reasons for why Bohr's atomic model falls apart. Check out: http://chemistry.tutorvista.com/inorga…/electron-shells.html

 RSS Feed
RSS Feed
